What's the difference between all of the different types of PAS platelet variations? Maybe you're only used to seeing one type, such as PAS-C, but there are other variations out there.
What are Platelet Additive Solution (PAS) platelets?
Well by actual volume, a whole-blood derived or apheresis platelet collection consists of mostly plasma. The platelets are suspended in the donor's plasma, ready for transfusion. To say "the blood type on a bag of platelets doesn't matter, just give the patient whatever" is not exactly true. I've heard this sentiment echoed in smaller hospitals and smaller blood banks where platelet inventory may not be as well stocked. You wouldn't think to give ABO incompatible plasma to a patient...right?
PAS Platelet Advantages
The major advantage to PAS platelets is the ability to transfuse out of type/ABO incompatible platelets to patients with a largely decreased risk of transfusion reaction. To accomplish this, 60-70% of the plasma is removed from the platelet donation and replaced with an isotonic crystalloid additive. This additive is devoid of ABO antibodies and other plasma proteins that could potentially cause an allergic reaction. ABO antibody titers are shown to be significantly reduced following this process. If your facility performs ABO titers on platelets already, perhaps PAS platelets can free up extra time in your daily process by no longer requiring titers to be performed on in-house platelet inventory. In many studies, HLA antibodies that were detected in non-PAS platelets, were now undetectable after undergoing PAS addition. This could potentially lower the risk of TRALI in patients as well.
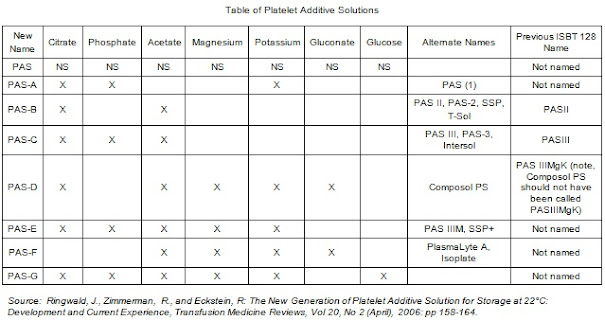
All of the additives contain normal saline (NS) for osmotic balance.
PAS-A -- Citrate, Phosphate, Potassium
PAS-B (T-SOL,SSP, PAS II) -- Citrate, Acetate (Removed Phosphate, K+, and added Acetate) Acetate was a key addition, as it helped with platelet shelf stability. It works in tandem with platelets active metabolism to ensure lactic acid does not build up during storage, as this would be detrimental. It does this by offering acetate as an energy source rather than glucose, as glycolysis would result in lactate, lowering pH levels. This product is not licensed in the USA.
PAS-C (PAS III/Intersol) -- Citrate, Phosphate, Acetate (Added phosphate). The re-addition of phosphate was key in adding extra buffering capability to the platelet solution. This keeps the pH of the solution stable as shelf life increases and the platelet storage lesion increases. Additionally this is helpful if the platelets were to undergo pathogen-inactivation, such as through the Intercept method. The Intercept method uses a compound known as Amotosalen-HCl. This could potentially acidify the environment, thus phosphate acts as a buffer to keep pH within a safe limit. An overly acidic environment would be detrimental to the platelets storage health. PAS-C is currently the most commonly used platelet additive solution in the US.
PAS-D (Composol PS) -- Citrate, Acetate, Magnesium, Potassium, Gluconate. The addition of Mg and K+ has shown diminished storage legion effects over the course of the platelet's shelf life and has a positive effect on inhibiting platelet aggregation/activation. pH was more stable as well as lactate production was lowered. Gluconate was added for its chelating abilities. It is thought that reducing the build of of calcium could result in lowered platelet activation and potentially even recover from early activation stages. Calcium is reduced by binding (chelating) with gluconate, usually in the form of Sodium gluconate.
PAS-E (PASIIIM/SSP+/T-PAS+) -- Citrate, Phosphate, Acetate, Magnesium, Potassium. What does SSP even stand for? It seems to be Safe (Platelets Through) Saved Plasma and is a trademark by MacoPharma. Manufacturers of PAS-E allow up to 80% plasma replacement with this product.
PAS-F (PlasmaLyte A, Isoplate) -- Acetate, Magnesium, Potassium, and Gluconate. This link provides a look into the actual packet insert of a platelet additive solution with all indications, warnings, etc.
PAS-G (M-SOL) -- Citrate, Phosphate, Acetate, Mg, Potassium, and Glucose. Although most civilians likely have never needed to deal with cryopreserved (frozen) platelets, they do still exist and perhaps better formations and technology can bolster more widespread usage of the product. Platelets with PAS-G added, saw the best platelet recovery percentage post thaw.
Although many PAS products share the same composition, the concentration of these additives may differ per dose.
Why not 100% PAS and no plasma? Well we're getting there! There is no perfect additive solution on the market today. Current in-use technologies require roughly 30% plasma to remain in solution. Anything less and platelets begin to suffer from quality issues. Newer PAS formulations are allowing for less and less plasma to be kept however. It is postulated that with the addition of glucose and other constituents such as bicarbonate (for buffering purposes), we can get very close to removing 100% of plasma from the platelet concentrate.